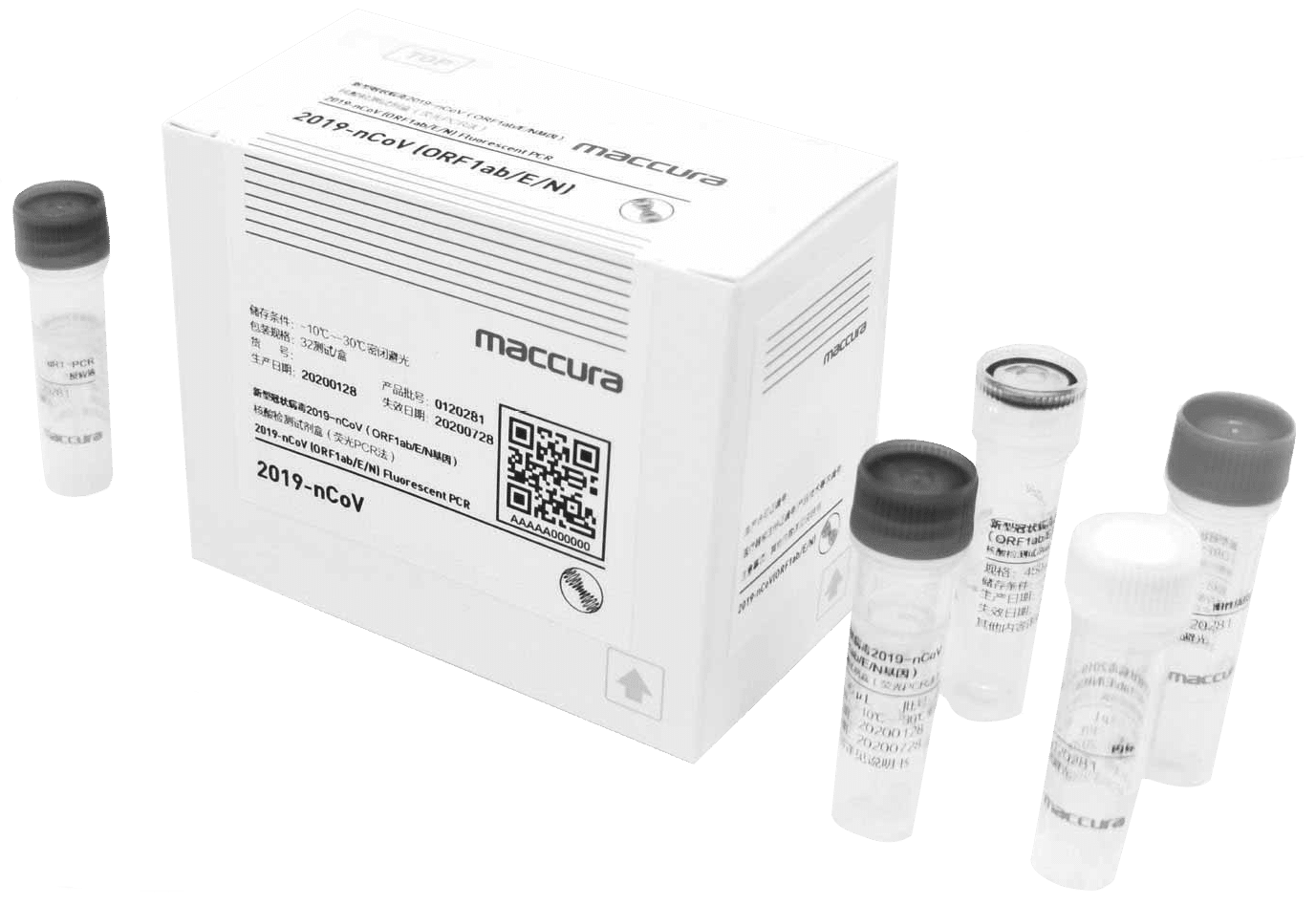
Usea combination of RT-PCR and qPCR techniques
Our COVID-19 Test Kits combine two Polymerase chain reaction (PCR)techniques – Real-time PCR (RT-PCR) and Reverse PCR (qPCR). The techniques are joined for a better and more accurate in-depth analysis of the viral RNA. With this combination, we have managed to negate the potential drawbacks of RT-PCR.
Multi-gene detection (ORF1a, ORF1b/RdRp, E, or N genes)
Depending on the Test Kit, different viral genes are targeted. This is done for maximum reliability of the tests. Please refer to the attached table for more information on which genes are targeted by which COVID-19 Test Kits.
| Product |
N gene |
E gene |
Orf1a |
Orf1b (RdRP) |
| GeneFinder™ COVID-19 Plus RealAmp Kit |
✓ |
✓ |
|
✓ |
| SARS-CoV-2 Fluorescent PCR |
✓ |
✓ |
|
✓ |
| DiaPlexQ Novel Coronavirus (2019-nCoV) Detection Kit |
✓ |
|
✓ |
|
| STANDARD™ M nCoV Real-Time Detection Kit |
|
✓ |
|
✓ |
Internal control reagents included
Provided in your COVID-19 Test Kits you will find vials containing internal control reagents. You can use them to verify the accuracy of the results at any time during the testing process. With them, you can be certain there was no contamination during any of the sample-handling stages.
Storage and Handling Requirements
The components of the kit should be stored at -20°C/-4°F or below until the expiration date stated. You shouldn’t use the kit if it seems defective. Please note that redundant freezing and thawing of the product might lead to inaccurate results.
CE-IVD marked, ISO 13485, FDA-cleared, Korea GMP-certified
Our COVID-19 tests are fully GMP and CE-certified, as shown by their respective labels on the product. Our viral COVID-19 tests are also compliant with the ISO 13485 standard. Please note that if you need reassurance regarding the certification of our products, we are willing to send you the copies of any relevant documents upon request!